Ensuring compliance with ISO 11607 is imperative in the field of packaging for terminally sterilized medical devices. The internationally harmonized standard is separated into two parts:
- ISO 11607 – 2 covers the requirements for the processes of forming, assembly and sealing of the packaging. The processes involved are known as a (packaging) process validation.
- ISO 11607 – 1 focuses on the requirements for the materials, sterile barrier system and packaging system. One of the main aspects of to be considered are the packaging system’s performance and stability. The processes involved are generally known as a packaging system design validation or simply packaging validation.
ISO 11607 – 2 – Process Validation
A process validation at a minimum must constitute of an installation qualification (IQ), an operational qualification (OQ) and a performance qualification (PQ). The general goal is to make sure that the equipment operates as intended and that the packaging can be produced as consistently, repeatably and reliably as expected.
Let’s take a closer look at the qualification phases:
- IQ – Installation Qualification
The purpose of Installation Qualification is to ensure that equipment, systems, and facilities are properly installed and meet the specified design criteria.
- OQ – Operational Qualification
OQ focuses on verifying and documenting that the equipment or system operates according to its operational specifications under normal operating conditions. For sealing processes this includes the determination of the lower process limits (LPL) and upper process limits (UPL) for critical process parameters like sealing temperature, time and pressure.
- PQ – Performance Qualification
This phase focuses on demonstrating that the equipment or system consistently performs according to its specified design and operational requirements in the intended operating environment. For sealing processes, packaging which were sealed at nominal process limits (NPL) are checked.
During the OQ and PQ, it is crucial to assess the effectiveness of the sealing through seal integrity testing, seal strength measurements and other packaging tests. See below for further information on a selection of these tests.
ISO 11607 – 1 – Material testing
Major parts of ISO 11607 – 1 focus on requirements for the materials used for the packaging system and sterile barrier systems, as well as for the packaging system and sterile barrier system themselves.
A selection of some of the most important requirements is listed in the following:
- Microbial barrier properties
Ensuring a microbial barrier is critical for ensuring integrity once sterilized, as it prevents the ingress of microorganisms.
- Biocompatibility
Biocompatibility should show that there are no critical substances that could migrate from the packaging material to the medical device and cause any harm for patients. Testing can involve cytotoxicity, nonvolatile residue etc.
- Physical and chemical properties
Conforming to a variety of physical and chemical properties ensures that the materials are suitable for the intended purpose of ensuring the overall product safety and especially ensuring the integrity even under physical stress during distribution. Testing for physical and chemical properties can for example involve burst testing, cleanliness, printing & coating, tensile properties, thickness etc. and have to conform to established properties and/or values.
- Sterilization compatibility
The materials used for the packaging system have to be compatible with the intended sterilization method (e.g., Gamma-ray, X‑Ray, EtO). Compatibility can for example be supported by testing of permeance, biocompatibility, seal strength and burst testing after exposure to the sterilization agent.
ISO 11607 – 1 – Packaging Validation
The main goal of a packaging validation is to establish evidence that the packaging system offers sufficient protection of the product, and, most importantly, that the sterile barrier system is capable of upholding its integrity after sterilization, distribution and storage.
A packaging system subjugated to a packaging validation should be manufactured under standard operating procedures using fully qualified equipment & sealing processes and sterilized using a validated sterilization process. In every aspect possible the worst-case scenario should be considered.
This includes:
- Worst-case medical device
If it is intended to use the same packaging system for a medical device product family (i.e., medical devices that are similar but not identical) the worst-case configuration out of this product family should be used. This can for example be the heaviest, bulkiest and/or sharpest configuration which poses the most stress on the packaging system.
- Worst-case sealing
Sterile barrier system (SBS) for the packaging validation should be sealed at LPL, which imposes the highest risk for compromising the integrity of the SBS.
- Worst-case sterilization
The samples for the packaging validation should be sterilized in a way to impose a higher challenge compared to the routine sterilization parameters. This for example can entitle a double-dose and/or a double-cycle sterilization.
A comprehensive packaging validation involves performance and stability testing. While it is not strictly prohibited to combine these two testing fields, it is recommended that they are kept separate from each other.
Packaging Validation – Performance Testing
Packaging performance testing involves a series of simulations and evaluations to ensure that the packaging system can withstand the challenges encountered during the expected handling and distribution.
Some important aspects to consider:
- Does the product ship as an individual box and/or as an unitized load on a pallet?
- Will the product ship as full truckload (FTL) or Less-Than-Truckload (LTL)
- In what climatic zones of the world will the product ship? What are the expected climatic conditions which could be encountered during transit?
- Will the product be shipped by boat, truck, rail and/or air?
Based on this information, it should be considered what the most appropriate consensus standard is that best aligns with the individual requirements.
Here is a selection of the most commonly used standards for packaging performance testing for medical device packaging:
- ASTM D4332
This standard is often used as a basis for testing environmental climatic conditions encountered during transit. Depending on which climatic zones the product will be shipped, the temperature can for example range from ‑40 °C to 70 °C and 10 % — 95% relative humidity. Knowledge of the expected climatic conditions during transit is essential, as these conditions should be tested in a series of simulated climatic conditioning steps which combined is known here at Früh as a climatic cycle.
- ASTM D4169
This widely used standard for transport simulations can be used as a basis to evaluate the ability of the packaging system to withstand the distribution environment with a focus on mechanical hazards, such as dropping, staking and vibration. Depending on the shipment configuration (single box vs. unitized load on a pallet) and expected shipment method (truck, rail, cargo ship and/or plane) the user can choose from a variety of different test sequences known as distribution cycles (DC).
- ISTA 3A
This standard shares many similarities with ASTM D4169 and is therefore another great option for transport simulations for medical device packaging. One important difference is that testing according to ISTA 3A can only be done on single boxes intended to be shipped via a parcel system. In general, testing according to this standard is more challenging but less customizable than its ASTM counterpart.
After performance testing, sterile barrier system integrity has to be proven through integrity testing. Seal strength measurements and other packaging tests can be included as well. See below for further information on a selection of these tests.
Packaging Validation – Stability Testing
The primary objective of stability testing is to assess the ability of the sterile barrier system to withstand the risks which can occur throughout the claimed shelf-life. The data from accelerated aging, which is aging conducted at elevated temperatures, is permissible to use for initial market launches, but the claimed shelf-life must be confirmed through real-time aging. ASTM F1980 is the go-to standard for establishing accelerated aging protocols for medical device packaging and gives guidance to calculate the time for the accelerated aging based on the Arrhenius equation.
Let’s take a look at an example:
An accelerated aging temperature of 55 °C, an assumed real-time aging temperature of 25 °C and an Arrhenius aging factor of Q10 = 2 allows for a reduction of the real-time aging time by a factor of 8. Therefore, for example, accelerated aging would shorten the time of 10 years real-time aging to 456.3 days (approximately 1.25 years).
After stability testing, sterile barrier system integrity has to be proven through integrity testing. Seal strength measurements and other packaging tests can be included as well. See below for further information on a selection of these tests.
Integrity and other packaging tests
Packaging systems including all sterile barrier systems have two very important functions in the life cycle of a terminally sterilized medical device:
- Protection of the medical device from damages until the point of use
- Ensuring sterility of the medical device until the point of use
Whether the packaging system is able to fulfill these functions has to be tested after each step of the packaging validation.
For this purpose, ISO 11607 – 1 lists a multitude of tests to choose from. Some of the most commonly used tests are listed below.
Integrity Tests:
- Bubble Emission Test through internal pressurization (ASTM F2096)
The packaging is placed underwater and subjected to increased internal pressurization. Constant bubble streams emerging from the packaging point to the existence of leaks.
- Bubble Emission Test – negative pressure (ASTM F3078)
The packaging is placed underwater in a chamber. A certain level of vacuum is applied to the chamber, which causes a pressure differential between the inside and outside of the package. Constant bubble streams emerging from the packaging point to the existence of leaks.
- Dye Penetration Test – porous packaging (ASTM F1929)
A dye solution is injected into the package, or, alternatively, the edges of each side of the seal are dipped into the dye solution. Each side of the seal seam is inspected for channels.
- Dye Penetration Test – non-porous packaging (ASTM F3039)
A dye solution is injected into the package. Each side of seal seam is then inspected for channels. Under certain conditions, it is additionally possible to inspect the flat surfaces of the packaging for leaks.
- Visual Inspection of the sealing (ASTM F1886/F1886M)
This method focuses on visually detecting channels and other quality relevant seal characteristic, such as undersealing, oversealing, folds etc.
Material Tests:
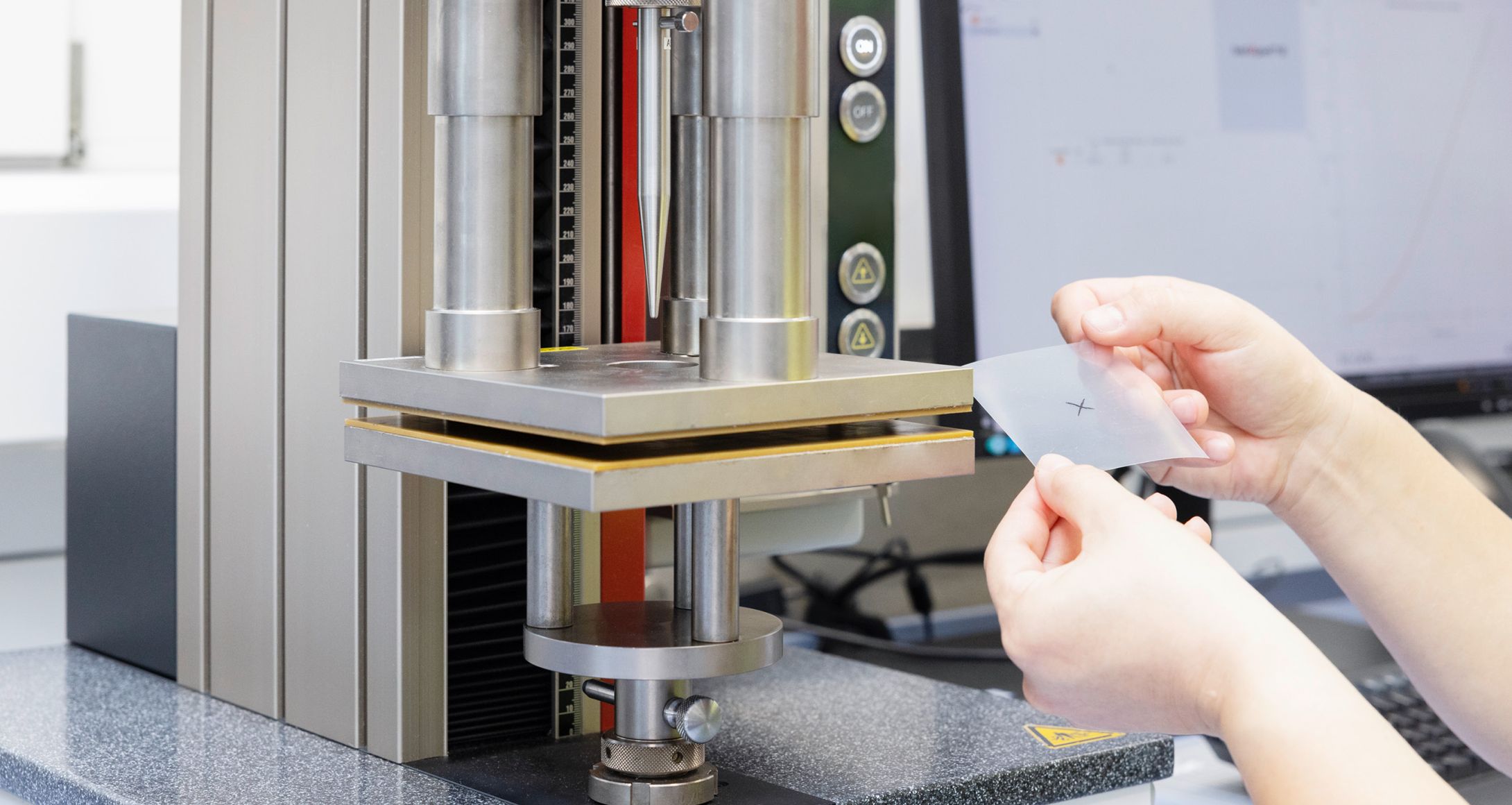
- Tensile Strength and Elongation at Break (DIN EN ISO 527−1÷527−3)
Tensile strength and elongation at break are two important mechanical properties used to characterize the behavior of materials under tension. Tensile strength is the maximum amount of tensile (stretching) stress that a material can withstand before failure or breaking. Elongation at break measures the extent to which a material can stretch or deform before it breaks. Both values are measured by pulling apart a strip of the test material.
- Puncture Resistance (DIN EN 14477 or ASTM F1306)
Puncture resistance is a mechanical property that measures a material’s ability to withstand the penetration of sharp objects without tearing or puncturing. The test is performed by forcing a probe into the material until complete penetration, resulting in the measurement of the force and energy at break.
Other packaging tests:
- Seal strength (ASTM F88/F88M)
The purpose of seal strength testing is to evaluate the ability of a seal to withstand the stress and pressure encountered during transportation, handling, and storage. For this test, a defined section of the seal seam is pulled apart until full separation, resulting in the measurement of the seal strength.
- Visual inspection of packaging (in-house method)
By visually examining packaging signs of damage, such as tears, punctures, or deformation, weaknesses and vulnerabilities in the packaging system can be identified. Investigating these issues helps to prevent product loss, contamination, or damage, ultimately safeguarding the sterile barrier system and the packaged product.
- Dimensional measurements (ASTM F2203 or in-house method)
Dimensional measurements can be taken from any part of the packaging. Most commonly this test is used to measure the seal width of the sterile barrier system either with a steel ruler (ASTM F2203) or with a digital caliper (in-house method).
- Category:
- Year:
- 2024